Describe the Units Used to Calculate Molarity
Definitions of solution solute and solvent. However it is now preferred following the unit SI system molkg or a similar SI unit.
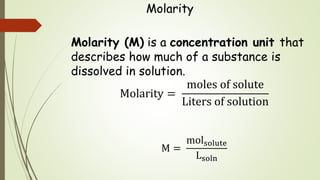
Molarity is the number of moles of solute per liter of solution.
. The SI unit for molar concentration is molm 3. Finally youre ready to determine molarity. Molalitys SI unit is molkg.
Mphantom rule 02em 0exfrac text mol solute text L solutionphantom rule 02em 0ex Calculating Molar Concentrations. It is equal to the moles of solute divided by the liters of solution. Only by observing which units are attached to a measurement can you determine whether youre working with molarity with mass percent or with a mass-mass mass-volume or volume-volume percent solution.
Molarity is also known as. Molarity is defined as the moles of a solute per liters of a solution. Molality is a solution property and is defined as the number of solvent moles per kilogram.
Remember to round to 4 significant digits. Units are our friends. The unit molL can be converted to molm 3 using the following equation.
How molarity is used to quantify the concentration of solute and calculations related to molarity. A solution that contains 1 mole of solute per 1 liter of solution 1 molL is called one Molar or 1 M. A mole is a numerical unitspecifically it represents the number of carbon atoms in a 1200 g mass of 12C and this number is N A Avogadros constant 602214085774 1023 mol1.
You know the end molarity 05 M NaOH which has units of moles volume You know the end volume 200 ml You should be able to calculate the number of moles of NaOH in the solution. Here s an example. Molarity moles litres.
The molality formula is given below. Molarity is defined as the number of moles of solute in exactly 1 liter 1 L of the solution. Molarity of solution mol KCL water.
Molarity is denoted by a capital M and M in chemistry means the following. Lets ask them for help. A solution with a 3 molarkg molality is often defined as 3 molal or 3 m.
The volume of the solution is essentially calculated in the unit of liters. Litres moles molarity. Calculate the molarity and the mass-volume percent solution obtained by dissolving 1029 g H 3 PO 4 into 642 mL final.
However molL is a more common unit for molarity. Molarity of the solution is the number of moles of the solute present in 1 liter of the solution. Moles volume end volume moles.
The molar mass of a monatomic element is the numerical value listed on the periodic table expressed in units of g. Simply express the concentration of KCl in water in terms of moles solute KCl per liters of solute water. For example the acetic acid here is completely dissolved in 125 L of water.
Molarity moles of solute litres of solution 0375 mol 0225 L 167 molL. 1 molL 1 moldm 3 1 mol dm 3 1 M 1000 molm 3. And the Periodic Table conveniently gives the mass associated with N A atoms of each element.
How do you calculate moles from molarity. Molarity Moles Solute Liter of Solution. If the solvent is water and the concentration of solute is fairly low ie dilute solution molality and molarity are approximately the same.
BeginarraylMolarity fracNumberofmolesofelementvolumeofsolutioninlitresendarray beginarraylMolality frac427times 10-2268times 10-2159Mendarray. Molarity M moles of solute liters of solution. Then divide 01665 moles by 125 L to get the molar concentration which will be 01332 M.
Left mathsf m right dfrac mathsf molesofsolute mathsf kilogramsofsolution. The solute is defined as the substance being dissolved while the solvent is the substance where the solute is dissolved usually water. Some students prefer to use a molarity triangle.
Liters of water 250 ml 1 L1000 ml liters of water 025 L. Molality is the number of moles of solute per kilogram of solvent. The unit to express molarity is moles per liter which is also called as molar.
If youre seeing this message it means were having trouble loading external resources on our website. The molality of a solution is calculated by taking the moles of solute and dividing by the kilograms of. Molarity 00161 mol KCl025 L water.
It summarizes the molarity formulas as. The most important concentration unit is molarity which is the number of moles of solute per liter of solution. Molality m and molarity M both express the concentration of a chemical solution.
MOLARITY number of moles volume in liters so if you have the volume along with the molarity you can calculate. Definition of Molarity M Molar Concentration or Molarity is defined as the number of moles of solute present in a definite amount of liters of the solution that is moles per liters of a solution. Now we calculate the molarity of the solution using the formula given above.
Molarity FormulaMolarity is the most commonly used term to describe the concentration of a solution. Molarity is symbolized by M. To calculate the Molar Concentration we will find the molar concentration by dividing the moles by liters of water used in the solution.
Molarity M is a useful concentration unit for many applications in chemistry. It is used to express the concentration of the solution. For example the molar mass of calcium Ca is with units of g.

Question Video Calculating The Molarity Of A Solution From Mass And Volume Nagwa

0 Response to "Describe the Units Used to Calculate Molarity"
Post a Comment